 After having been the editor of the Ethics Blog for eight years, I would like to describe the research communication that usually occurs on this blog.
After having been the editor of the Ethics Blog for eight years, I would like to describe the research communication that usually occurs on this blog.
The Ethics Blog wants to avoid the popular scientific style that sometimes occurs in the media, which reports research results on the form, “We have traditionally believed that…, but a recent scientific study shows that…” This is partly because the Ethics Blog is run by a research center in ethics, CRB. Although ethics may involve empirical studies (for example, interviews and surveys), it is not least a matter of thinking. If you, as an ethicist, want to develop new recommendations on informed consent, you must think clearly and thoroughly. However, no matter how rigorously you think, you can never say, “We have traditionally believed that it is ethically important to inform patients about…, but recent philosophical thoughts show that we should avoid doing that.”
Thinking does not provide the authority that empirical research gives. As an ethicist or a philosopher, I cannot report my conclusions as if they were research results. Nor can I invoke “recent thoughts” as evidence. Thoughts give no evidence. Ethicists therefore present their entire thinking on different issues to the critical gaze of readers. They present their conclusions as open suggestions to the reader: “Here is how I honestly think about this issue, can you see it that way too?”
The Ethics Blog therefore avoids merely disseminating research results. Of course, it informs about new findings, but it emphasizes their thought provoking aspects. It chooses to reflect on what is worth thinking about in the research. This allows research communication to work more on equal terms with the reader, since the author and the reader meet in thinking about aspects that make both wonder. Moreover, since each post tries to stand on its own, without invoking intellectual authority (“the ethicists’ most recent thoughts show that…”), the reader can easily question the blogger’s attempts to think independently.
In short: By communicating research in a philosophical spirit, science can meet people on more equal terms than when they are informed about “recent scientific findings.” By focusing on the thought provoking aspects of the research, research communication can avoid a patronizing attitude to the reader. At least that is the ambition of the Ethics Blog.
Another aspect of the research communication at CRB, also beyond the Ethics Blog, is that we want to use our ordinary language as far as possible. Achieving a simple style of writing, however, is not easy! Why are we making this effort, which is almost doomed to fail when it comes to communicating academic research? Why do Anna Holm, Josepine Fernow and I try to communicate research without using strange words?
Of course, we have reflected on our use of language. Not only do we want to reach many different groups: the public, patients and their relatives, healthcare staff, policy makers, researchers, geneticists and more. We also want these groups to understand each other a little better. Our common language accommodates more human agreement than we usually believe.
Moreover, ethics research often highlights the difficulties that different groups have in understanding each other. It can be about patients’ difficulties in understanding genetic risk information, or about geneticists’ difficulties in understanding how patients think about genetic risk. It may be about cancer patients’ difficulties in understanding what it means to participate in clinical trials, or about cancer researchers’ difficulties in understanding how patients think.
If ethics identifies our human difficulties in understanding each other as important ethical problems, then research communication will have a particular responsibility for clarifying things. Otherwise, research communication risks creating more communication difficulties, in addition to those identified by ethics! Ethics itself would become a communication problem. We therefore want to write as clearly and simply as we can, to reach the groups that according to the ethicists often fail to reach each other.
We hope that our communication on thought provoking aspects of ethics research stimulates readers to think for themselves about ethical issues. Everyone can wonder. Non-understanding is actually a source of wisdom, if we dare to admit it.
Pär Segerdahl
This post in Swedish







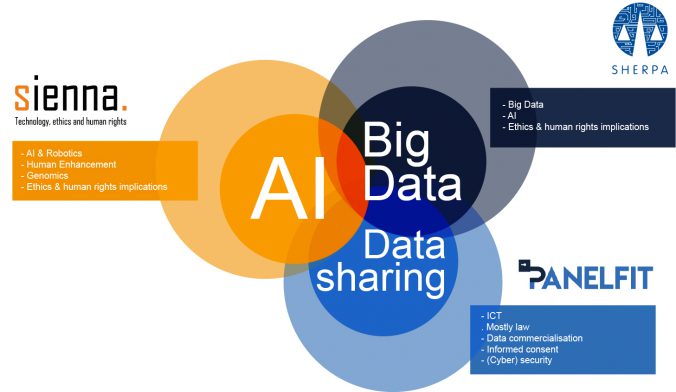
 Do you use Google Maps to navigate in a new city? Ask Siri, Alexa or OK Google to play your favourite song? To help you find something on Amazon? To read a text message from a friend while you are driving your car? Perhaps your car is fitted with a semi-autonomous adaptive cruise control system… If any software or machine is going to perform in any autonomous way, it needs to collect data. About you, where you are going, what songs you like, your shopping habits, who your friends are and what you talk about. This begs the question: are we willing to give up part of our privacy and personal liberty to enjoy the benefits technology offers.
Do you use Google Maps to navigate in a new city? Ask Siri, Alexa or OK Google to play your favourite song? To help you find something on Amazon? To read a text message from a friend while you are driving your car? Perhaps your car is fitted with a semi-autonomous adaptive cruise control system… If any software or machine is going to perform in any autonomous way, it needs to collect data. About you, where you are going, what songs you like, your shopping habits, who your friends are and what you talk about. This begs the question: are we willing to give up part of our privacy and personal liberty to enjoy the benefits technology offers.


Recent Comments