Coercive measures against patients are regularly used in healthcare outside of psychiatry, for example in neurosurgical care. Examples of such measures are belting, boxing gloves, holding patients down, forced medication and hidden medication. It is mostly nurses who carry out these coercive measures. The most common motive for forcing patients is to protect them from harming themselves or others: patients may be confused or aggressive and try to pull out vital ports for intravenous drug administration or abuse staff, often without understanding what they are doing themselves. Because the staff act in a legal and moral gray zone, they often feel moral stress exercising coercion.
How can we regulate coercive care in a way that balances ethically relevant considerations about the measures, so that staff no longer have to act in a gray zone?
Different countries have chosen different paths to regulate coercive care within somatic healthcare. In Sweden, it is in principle illegal to use all forms of coercion without the support of compulsory psychiatric care. An overarching problem in the regulation of coercive care is to ensure that patients with reduced decision-making capacity receive the care they need and at the same time ensure that patients with a sufficient degree of decision-making capacity are not forced into care they do not want. In an article in the Journal of Medical Ethics, Amina Guenna Holmgren and I and two co-authors try to sort out these difficulties. Arguments about justice, trust in healthcare, minimizing harm and respect for autonomy are made for and against different national regulations. We conclude that a regulation that includes an assessment of the patients’ decision-making capacity and takes the patient’s best interests into account is preferable, in contrast to regulations based on psychiatric diagnoses or regulations where there are no legal possibilities to practice coercive care at all within somatic care.
If you want to take a closer look at our reasoning regarding the regulation of restraint in somatic healthcare and evaluate our proposal, you will find the article here: Restraint in somatic healthcare: how should it be regulated?

Written by…
Niklas Juth, Professor of Clinical Medical Ethics at the Centre for Research Ethics & Bioethics (CRB)
Guenna Holmgren A, von Vogelsang A, Lindblad A, Niklas Juth. Restraint in somatic healthcare: how should it be regulated? Journal of Medical Ethics. Published Online First: 18 October 2023. doi: 10.1136/jme-2023-109240
Thinking about law



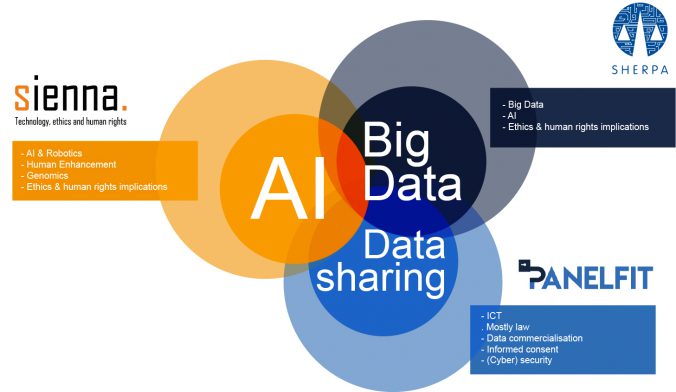
 Do you use Google Maps to navigate in a new city? Ask Siri, Alexa or OK Google to play your favourite song? To help you find something on Amazon? To read a text message from a friend while you are driving your car? Perhaps your car is fitted with a semi-autonomous adaptive cruise control system… If any software or machine is going to perform in any autonomous way, it needs to collect data. About you, where you are going, what songs you like, your shopping habits, who your friends are and what you talk about. This begs the question: are we willing to give up part of our privacy and personal liberty to enjoy the benefits technology offers.
Do you use Google Maps to navigate in a new city? Ask Siri, Alexa or OK Google to play your favourite song? To help you find something on Amazon? To read a text message from a friend while you are driving your car? Perhaps your car is fitted with a semi-autonomous adaptive cruise control system… If any software or machine is going to perform in any autonomous way, it needs to collect data. About you, where you are going, what songs you like, your shopping habits, who your friends are and what you talk about. This begs the question: are we willing to give up part of our privacy and personal liberty to enjoy the benefits technology offers.



Recent Comments